Details of the Drug
General Information of Drug (ID: DMKP6BM)
| Drug Name |
2-Methylamino-succinic acid(NMDA)
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
N-Methyl-D-aspartic acid; N-methyl-D-aspartic acid; NMDA; 6384-92-5; N-Methyl-D-aspartate; N-Methylaspartate; D-Aspartic acid, N-methyl-; N-Me-D-Asp-OH; (R)-2-(Methylamino)succinic acid; NMDA (N-Methyl-D-aspartic acid); Methyl aspartic acid; n-methyl-d-aspartic acid (nmda); UNII-1903B9Q6PI; N-Methyl aspartic acid; (2R)-2-(methylamino)butanedioic acid; 2-Methylamino-succinic acid; BRN 1724431; CHEMBL291278; CHEBI:31882; HOKKHZGPKSLGJE-GSVOUGTGSA-N; 1903B9Q6PI; N-Methyl-D-aspartic Acid, Hydrate; AK-44365; W-203368; N-Methyl-D-aspartic acid, 98%
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
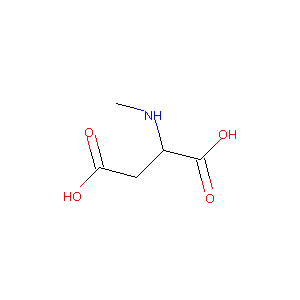 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 147.13 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
